 Many people ask me which is the best magnesium (Mg) supplement to take and I always answer Mg Chloride (MgCl2). Then there’s the inevitable second question. What is Mg Chloride? Before I can answer there is often a 3rd question. Why do you recommend Mg Chloride rather than any other Mg supplement?
Many people ask me which is the best magnesium (Mg) supplement to take and I always answer Mg Chloride (MgCl2). Then there’s the inevitable second question. What is Mg Chloride? Before I can answer there is often a 3rd question. Why do you recommend Mg Chloride rather than any other Mg supplement?
So this article is to fully explain what MgCl2 is and why I think it is the best Mg supplement to take. Have a look at this article about the benefits of this supplement.
Mg Chloride is the name for the chemical compound with the formula MgCl2 and its various hydrates MgCl2(H2O)x. These salts are highly soluble in water. The hydrated Mg Chloride can be extracted from brine or sea water.
So many variables
The problem with Mg is, there are so many variations with some Mg salts being more bio available than others. But who do you trust to tell you the truth? The pharmaceutical companies? The food manufacturers? Your doctor? It’s no wonder the public is confused as to which is the most efficient and bio available. This is apart from the fact that few in the medical industry seem to even know about Mg and that includes allopathic doctors!
 Not sure?
Not sure?
If you’re not sure what to do, research for yourself and your family. If you haven’t got time to do your own research, look for a source that has no axe to grind and gives out information freely. A source that has no ties with big pharma, big agra or food manufacturers.
A list of different Mg salts
Listed below in alphabetical order, is a rundown of the different magnesium salts available. You have probably realised that you can’t take magnesium in its elemental form ie. as a metal so it has to be processed to make it available to the body as a supplement.
- Magnesium Aspartate – SCV 2.43 – Avoid – breaks down to aspartic acid which is neurotoxic
- Magnesium Bicarbonate – used as antacids
- Magnesium Carbonate – used as antacids
- Magnesium Chloride (6H2O) – SCV zero – 120mg of elemental Mg per 1 gram of salt
- Magnesium Citrate – SCV 2.8 – 150mg of elemental Mg per 1 gram of salt
- Magnesium Gluconate – SCV 0.70 – 50mg of elemental Mg per 1 gram of salt – used in drips
- Magnesium Glutamate – Avoid – breaks down to glutamic acid which is neurotoxic
- Magnesium Glycinate – SCV 3.45 – 100mg of elemental Mg per 1 gram of salt
- Magnesium Lysinate – used as a food additive
- Magnesium Malate – SCV 1.55 – 150mg of elemental Mg per l gram of salt
- Magnesium Orotate – 60mg of elemental Mg per 1 gram of salt
- Magnesium Oxide – Only 4% bio available – 600mg of elemental Mg per 1 gram of salt

- Magnesium Phosphate – laxatives and antacids
- Magnesium Sulfate – Epsom Salts – Laxative – 100mg of elemental Mg per 1 gram of salt – used in drips
- Magnesium Taurate – 100mg of elemental Mg per 1 gram of salt
It’s all about the bond
You may have noticed there is an SCV number next to some of them. This is the Stability Constant Value of the Mg salts. This tells you if the strength of the bond between the two components is strong or weak. The higher the number, the stronger the bond and the less bio availability.
Mg oxide’s laxative effect
Mg Oxide, for instance, has a very strong bond between the Mg and the oxygen which means it will not dissociate easily so the Mg cannot be used to any great extent biologically. Instead, most of the compound will go straight through you giving a laxative  effect. OK if you want a clear out! I cannot find the official SCV of Mg oxide or some of the other salts, but I know the SCV of Mg oxide is high and is therefore strongly bonded to oxygen. Unfortunately, Mg oxide is commonly added as the dietary source of magnesium to foods and supplements, because it’s cheap. This likely produces dietary deficiencies resulting in poor health and a reduced life span. Mg oxide is at most only 4% bio available.
effect. OK if you want a clear out! I cannot find the official SCV of Mg oxide or some of the other salts, but I know the SCV of Mg oxide is high and is therefore strongly bonded to oxygen. Unfortunately, Mg oxide is commonly added as the dietary source of magnesium to foods and supplements, because it’s cheap. This likely produces dietary deficiencies resulting in poor health and a reduced life span. Mg oxide is at most only 4% bio available.
MgCl2 stability constant of zero
As you can see, Mg Chloride (MgCl2) has an SCV of zero. This means the bond between the Mg and the chlorine to produce Mg Chloride is very weak. This is good, because the two quickly disassociate from each other leaving the Mg in its ionic state to do its work immediately within the body.
What about Mg Citrate?
Mg Citrate also has quite a low SCV of just 2.8 and has good bio availability. This molecule will give you a gentle laxative effect if you suffer from constipation which is a symptom for some people who have a Mg deficiency. It is also very good for children, who often suffer with constipation, mainly because of all the junk food that’s available to them. This is a very distressing condition for a child as well as the parents and also means that child has a Mg deficiency which can cause all kinds of serious conditions such as ADHD, ADD and asthma to name but a few and if not addressed, heralds health problems for the child’s future. See this article which covers child constipation.
Food manufacturers at fault
You can blame the food manufacturers for putting temptation in a youngsters way with all the relentless advertising of all the sweets and processed foods such as cereals, biscuits and fizzy drinks. Parents will generally relent when being nagged constantly by their child to buy certain items of sweets, junk food or carbonated drinks.
Mg Citrate can be bought in a tasteless powder form which you can put into soups, porridges etc.. The kids won’t even know it’s there. With Mg Chloride such as ReMag, the taste can be salty and bitter, especially if not taken with enough water. Kids will often baulk at taking it unless it is well disguised (please, not with aspartame laced fizzy drinks).
Mg Gluconate in hospital drips
The other low SCV supplement is Mg Gluconate at SCV 0.70. Have you ever visited a hospital, particularly to see an older friend or relative. The patient is often attached to a clear fluid drip for re-hydration. If you look at the contents of the drip bag (without touching it of course) you will likely see Mg Gluconate as one of the salts.
 Mg Sulphate & Pregnancy
Mg Sulphate & Pregnancy
Mg Sulphate is a treatment for preeclampsia, a condition of pregnancy. This dangerous problem can be avoided if the mother is well supplemented with Mg before and during her pregnancy. The lack of this vital mineral can kill. That does sound a little dramatic but this is a condition that can be a danger to both mother and child and has also been linked to SIDS (sudden infant death syndrome).
Naming salts
The name of a salt has two parts. The first part is the name of the metal and second is the salt that is formed. The second part of the name comes from the acid used to make it. The names of salts made from hydrochloric acid end in -chloride, while the names of salts made from sulfuric acid end in -sulfate.
Formation of salts
| Metal | Acid | Salt | ||
|---|---|---|---|---|
| aluminium | reacts with | hydrochloricacid | to make | aluminium chloride |
| copper | reacts with | hydrochloricacid | to make | copper chloride |
| calcium | reacts with | sulfuric acid | to make | calcium sulfate |
| zinc | reacts with | sulfuric acid | to make | zinc sulfate |
Mg forms Mg salts when it reacts with acids. Therefore, Mg reacts with hydrochloric acid to make Mg chloride
So why is Mg Chloride so bio available
- Mg is dissociated from the weak chloride bond, leaving a Mg ion that is so small it will readily permeate through cell walls. Because of this permeability, the Mg does not cause the laxative effect because much of it does not get into the gut before it has been absorbed into the body.
- The compound MgCl2 is a very small molecule, consequently it can even be absorbed through the skin, that’s why many people use it topically. (Another topical application is Mg sulphate (epsom salts), which people often put in their bathwater for a relaxing soak.) Great for getting more Mg into your body whilst pregnant
 MgCl2 supplements on the market
MgCl2 supplements on the market
There are a few MgCl2 supplements on the market, perhaps the most famous is Dr Carolyn Dean’s ReMag, a very popular and high quality supplement. Dr Dean advertises her product concentrating on the tiny size of the molecule, calling it ‘picometer’ size. This well explains how very small this compound is. It could be misconstrued that the picometer size is only applicable to this particular brand of MgCl2 but this is not the case. Whichever brand you use, all the supplements will be this very small ‘picometer’ size and this is the beauty of MgCl2
Dr Carolyn Dean’s ReMag
ReMag is easily available in the US where it is competitively priced, not so in the UK, where it is very expensive. This makes it difficult for those with limited funding to afford. That being the case, if you really want to use MgCl2 you can make it up yourself.
You must, however obtain the best quality MgCl2 salt to make into your solution. Always use food quality, don’t be tempted to go for the cheaper lower quality stuff. Once you have obtained your MgCl2 from a reputable supplier, you can make up a 250ml bottle of the solution as follows:
 How to make up MgCl2 solution
How to make up MgCl2 solution
Put 125 grams into a small marked pyrex jug. Add just a little mineral water, enough to dissolve the salts and stir. Add more water to make your solution up to the 250ml mark, stir again and then pour it into a suitable glass bottle. It will be very cloudy at first but that is not sediment, it is air bubbles, which will start to clear in a few minutes.
100 doses from 250ml
This will give you 100 doses of 2.5ml (½ teaspoon). Put one dose into a glass of water and drink over the course of the day. If you don’t like the taste, lace it with a little cordial (NOT low sugar, we don’t want aspartame in it). The taste doesn’t bother me but some find it a little bitter (hubby uses Rose’s lime cordial). Up your doses gradually until you feel the difference. Always spread your doses throughout the day. Everyone needs a different dose and it’s a case of finding your own level. I take around 2 teaspoons in a litre of water and drink it slowly throughout the day. This gives me the equivalent of 600mg of elemental Mg. I used to suffer from insomnia, so to stop it recurring, I drink at least a quarter of my daily dose in the evening. If I’m stressed or have a heavy day ahead, I will up my dose from 2 teaspoons to 2½.
Making up your own MgCl2 solution is much cheaper than buying it ready made. It is a little inconvenient but well worth the trouble. I have never bought MgCl2 in solution, I am quite happy  making it up myself. Mind you, it is quite useful having hubby around as he has qualifications in peptide chemistry and has worked in a laboratory environment as a senior technical officer.
making it up myself. Mind you, it is quite useful having hubby around as he has qualifications in peptide chemistry and has worked in a laboratory environment as a senior technical officer.
If someone who is au fait with chemistry takes this supplement every day, it may give you a clue as to how important it is for your overall health.
 To finish off this 4 part article Drugs! are you on a diuretic, I will show you some questions written by worried patients prescribed diuretics. If you’re on this class of drugs, you may find a question here relating to you and problems you are encountering. As Furosemide is a diuretic that is one of the most prescribed drugs in the world, questions about this particular drug will be covered here.
To finish off this 4 part article Drugs! are you on a diuretic, I will show you some questions written by worried patients prescribed diuretics. If you’re on this class of drugs, you may find a question here relating to you and problems you are encountering. As Furosemide is a diuretic that is one of the most prescribed drugs in the world, questions about this particular drug will be covered here.  Furosemide (Lasix) – A loop diuretic
Furosemide (Lasix) – A loop diuretic about this drug. My husband fortunately works close to home; he sometimes urinates on himself because he can’t always make it to the bathroom in time. He does operate heavy equipment at times and the dizziness thing is scary too. Please tell us more about furosemide (Lasix) side effects.”
about this drug. My husband fortunately works close to home; he sometimes urinates on himself because he can’t always make it to the bathroom in time. He does operate heavy equipment at times and the dizziness thing is scary too. Please tell us more about furosemide (Lasix) side effects.”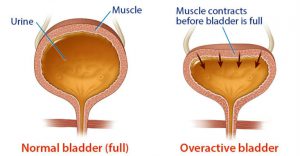 Overactive bladder
Overactive bladder whilst suffering the side effects of dizziness or vertigo is an accident waiting to happen. This just confirms that this doctor failed in his duty to make sure his patient had the best possible medication for his particular lifestyle. This is a dereliction of duty in my mind, apart from the obvious misery it is causing the patient and his wife.
whilst suffering the side effects of dizziness or vertigo is an accident waiting to happen. This just confirms that this doctor failed in his duty to make sure his patient had the best possible medication for his particular lifestyle. This is a dereliction of duty in my mind, apart from the obvious misery it is causing the patient and his wife.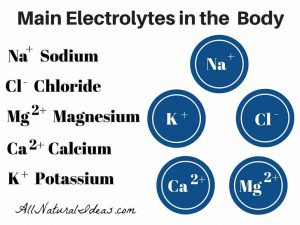 “My mother had swelling in her ankles, she only weighed 74 lbs. Her Dr. prescribed her lasix 40 mg.
She took one time day. We had home health nursing too. Mom was feeling weaker loss of appetite. They couldn’t figure out what’s wrong. About week Mom says let’s go to Hosp. we waiting for results, her body had no sodium.
Dr. didn’t prescribe potassium with her lasix…we were in Hosp 2 wks . We were home 2wks. She never recovered from that. I lost her…do u believe Dr. is responsible…I do.”
“My mother had swelling in her ankles, she only weighed 74 lbs. Her Dr. prescribed her lasix 40 mg.
She took one time day. We had home health nursing too. Mom was feeling weaker loss of appetite. They couldn’t figure out what’s wrong. About week Mom says let’s go to Hosp. we waiting for results, her body had no sodium.
Dr. didn’t prescribe potassium with her lasix…we were in Hosp 2 wks . We were home 2wks. She never recovered from that. I lost her…do u believe Dr. is responsible…I do.”
 Are doctors in control?
Are doctors in control?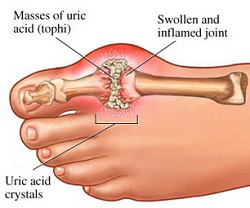
 The possibility of hearing loss, which can be irreversible, is on the list of side effects for this drug. It should be stressed by all doctors, the serious side effects this drug can cause including kidney damage and irreversible hearing loss and many more. I’m sure if this patient had been told about the side effects, she would have requested a different medication. But she hasn’t been given the option. Because of this lack of information, she could be permanently hearing impaired!
The possibility of hearing loss, which can be irreversible, is on the list of side effects for this drug. It should be stressed by all doctors, the serious side effects this drug can cause including kidney damage and irreversible hearing loss and many more. I’m sure if this patient had been told about the side effects, she would have requested a different medication. But she hasn’t been given the option. Because of this lack of information, she could be permanently hearing impaired! Husband changed into a ‘Zombie’
Husband changed into a ‘Zombie’ something else instead and reducing the amounts of other drugs, my husband miraculously became alive again. He is now 86 years old and has survived two life-threatening surgeries. He is now very active and building things like he used to that he loved to do. He does many tasks and does not want to sleep all day, eats well and does so many other things…
something else instead and reducing the amounts of other drugs, my husband miraculously became alive again. He is now 86 years old and has survived two life-threatening surgeries. He is now very active and building things like he used to that he loved to do. He does many tasks and does not want to sleep all day, eats well and does so many other things… Prime example of over prescribing
Prime example of over prescribing From the horse’s mouth!
From the horse’s mouth!
 “I have these cramps so bad and gout, now I see it came from the Lasix as a side effect and my doctor just upped my dosage to 40 from 20. I am swollen in the legs and feet and I think my water pill isn’t working. I don’t think I’ll up the dosage but stop generic brand and opt for another brand of water pill. These doctors are not telling us everything. The gout is not hereditary, it came from the water pill and so did the ringing in my ears and blurry vision.”
“I have these cramps so bad and gout, now I see it came from the Lasix as a side effect and my doctor just upped my dosage to 40 from 20. I am swollen in the legs and feet and I think my water pill isn’t working. I don’t think I’ll up the dosage but stop generic brand and opt for another brand of water pill. These doctors are not telling us everything. The gout is not hereditary, it came from the water pill and so did the ringing in my ears and blurry vision.”