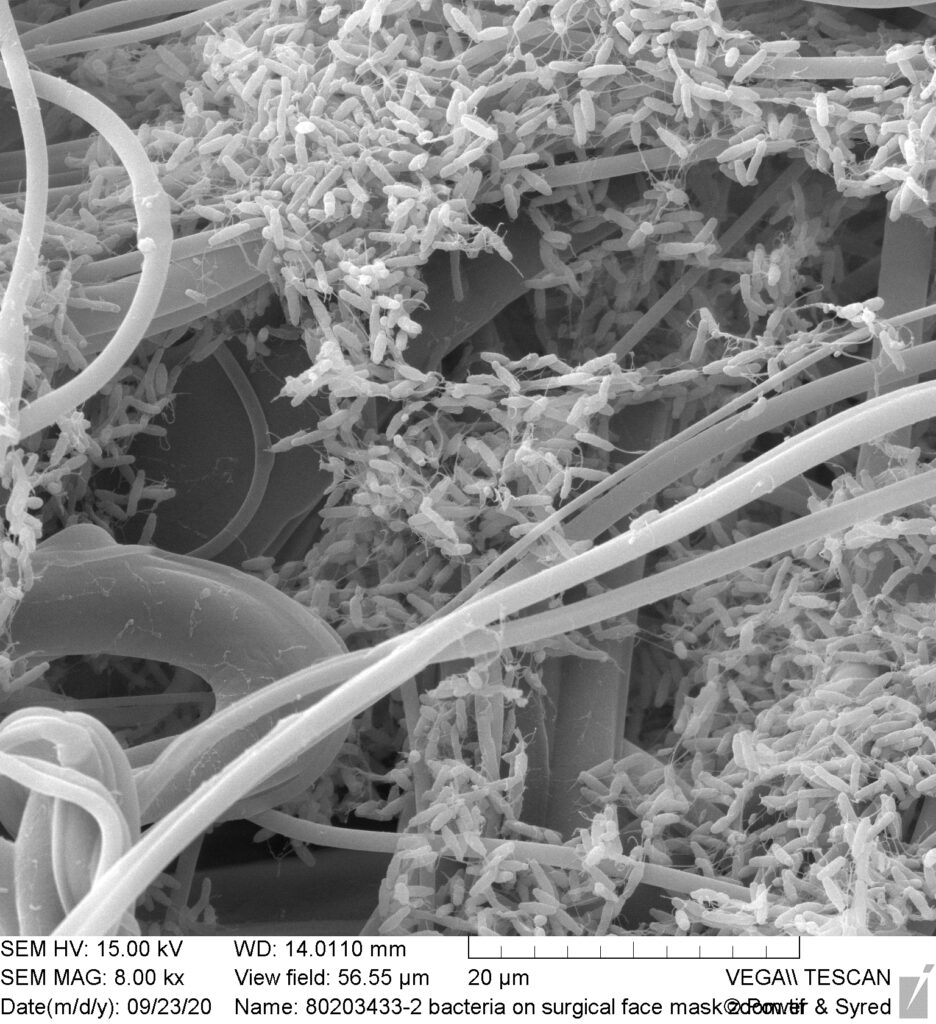
This is a micrograph of a one day old surgical face mask. We used our Scanning Electron Microscope at a magnification of x8,000. The width of this micrograph represents just 0.05mm (1/20th millimetre). To make this minuscule size easier to comprehend, the width of this image is 10 times smaller than any of the full stops on the data bar under the micrograph. At that size 100 of these images could be contained within the whole full stop ie. 10 x 10 = 100; the full stop is actually square.
 Even more minuscule
Even more minuscule
If a full stop was ½mm square, 4,000 SARS-CoV-2 viruses would fit across it. To cover the whole full stop with just one layer, would take 16,000,000 viruses. Do you really think your mask will protect you? Why do virologists wear a Hazmat suit?
Masks lower your immune system, cause you stress, burden your lungs with excess pathogens and exacerbate any medical problems you have already. As for putting masks on children, I personally think it’s child abuse!
How many pathogens, millions…billions?
Imagine the accumulation of bacteria and other pathogens on the whole of this mask! How many people are continually breathing these back into their lungs. A moist mask is a breeding ground; look at the build-up after just one day, on an area 10x smaller than the width of a full stop!
Be Aware!
Please be aware of the harm wearing a mask can do to your physical and psychological health. The information below gives you more details. If you are uncomfortable or stressed or have any reason, physical or psychological, you are exempt from wearing a mask. Nobody has the right to ask you why you’re not wearing a mask. “I am exempt” is all you need say.
Taken from the www.gov.uk website – face coverings
If you have an age, health or disability reason for not wearing a face covering:
- You do not routinely need to show any written evidence of this.
- You do not need show an exemption card.
- You do not need to seek advice or request a letter from a medical professional about your reason for not wearing a face covering.
- Some people may feel more comfortable showing something that says they do not have to wear a face covering. This could be in the form of an exemption card, badge or even a home-made sign.
- Carrying an exemption card or badge is a personal choice. It is not required by law.
More Information on Face Masks (courtesy of Dr Joe Mercola article)
Wearing a face mask increases breathing resistance, and since it makes both inhaling and exhaling more difficult, individuals with pre-existing medical conditions may be at risk of a medical emergency if wearing a face mask.
This includes those with shortness of breath, lung disease, panic attacks, breathing difficulties, chest pain on exertion, cardiovascular disease, fainting spells, claustrophobia, chronic bronchitis, heart problems, asthma, allergies, diabetes, seizures, high blood pressure and those with pacemakers. The impact of wearing a face mask during pregnancy is also wholly unknown.
Face masks can reduce oxygen intake, leading to potentially hazardous oxygen deficiency (hypoxia).
 They also cause rapid accumulation of harmful carbon dioxide, which can have significant cognitive and physical impacts. Germany’s first registry. 13,14 recording the effects mask wearing has on children, has identified 24 physical, psychological and behavioural health issues associated with wearing masks. Recorded symptoms include:
They also cause rapid accumulation of harmful carbon dioxide, which can have significant cognitive and physical impacts. Germany’s first registry. 13,14 recording the effects mask wearing has on children, has identified 24 physical, psychological and behavioural health issues associated with wearing masks. Recorded symptoms include:
“… irritability (60%), headache (53%), difficulty concentrating (50%), less happiness (49%), reluctance to go to school/kindergarten (44%), malaise (42%), impaired learning (38%) and drowsiness or fatigue (37%).”
Of the 25,930 children included in the registry, 29.7% reported feeling short of breath, 26.4% being dizzy and 17.9% were unwilling to move or play. Hundreds more experienced “accelerated respiration, tightness in chest, weakness and short-term impairment of consciousness.”
Wearing a face mask increases your body temperature and physical stress, which could result in an elevated temperature reading that is not related to infection.
All face masks can cause bacterial and fungal infections in the user as warm, moist air accumulates inside the mask. This is the perfect breeding ground for pathogens. This is why disposable medical masks were designed for short-duration, specific-task use only, after which they are supposed to be discarded.
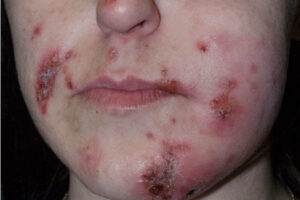 Medical doctors have warned that bacterial pneumonia, facial rashes, fungal infections on the face15 “mask mouth” (symptoms of which include bad breath, tooth decay and gum inflammation) and candida mouth infections16 are all on the rise.
Medical doctors have warned that bacterial pneumonia, facial rashes, fungal infections on the face15 “mask mouth” (symptoms of which include bad breath, tooth decay and gum inflammation) and candida mouth infections16 are all on the rise.
A study17,18 published in the February 2021 issue of the journal Cancer Discovery also found that the presence of microbes in your lungs can worsen lung cancer pathogenesis and can contribute to advanced stage lung cancer. The same types of bacteria, primarily Veillonella, Prevotella, and Streptococcus bacteria, can also be cultivated through prolonged mask wearing. 19
With extended use, medical masks will begin to break down and release chemicals that are then inhaled. Tiny microfibers are also released, which can cause health problems when inhaled. This hazard was highlighted in a performance study20 being published in the June 2021 issue of Journal of Hazardous Materials.
Your rights
Nobody has the right to mandate you restrict your breathing and cause you potential harm. It must be your decision as to whether or not you wear a face mask. Mandatory wearing of face masks is an affront to civil liberties and can be damaging physically and psychologically. Do not be terrorised or intimidated, it’s your body, your health.
References:
- 1, 2, 3, 4 New York Times April 22, 2021 (Archived)
- 5 Nature Communications November 20, 2020; 11 Article number 5917
- 6 The New York Times April 27, 2021
- 7 Environmental Research February 2021; 193: 110603
- 8 MedRxiv March 3, 2020 DOI: 10.1101/2020.02.28.20029272
- 9 Nature June 2020; 582(7813):557-560
- 10 Preprints May 29, 2020: 202005464
- 11 Environ Int January 2021; 146: 106255
- 12 Todayville June 2020
- 13 Research Square, 2021; doi.org/10.21203/rs.3.rs-124394/v2
- 14 Montana Daily Gazette, January 25, 2021
- 15 Global Research January 21, 2021
- 16 The Crimson White August 20, 2020
- 17 Cancer Discovery February 2021 DOI: 10.1158/2159-8290.CD-20-0263
- 18 AZO Life Sciences November 12, 2020
- 19 Global Research February 3, 2021
- 20 Journal of Hazardous Materials June 5, 2021; 411: 124955
- 21 Journal of Orthopaedic Translation July 2018; 14: 57-62
- 22 Newsweek April 6, 2021
- 23 Fox News February 23, 2021
- 24 Prairie Public February 23, 2021
- articles.mercola.com
 First of All – Turn Off Mainstream Media
First of All – Turn Off Mainstream Media 2. Children’s education
2. Children’s education 6. Jobs
6. Jobs 11. Weddings
11. Weddings 17. Going to Restaurants
17. Going to Restaurants 21. Our Smiles
21. Our Smiles
 Not all of us are losing!
Not all of us are losing! Celebrities
Celebrities

 Dr. James Meehan, MD warned that mask wearing has..
Dr. James Meehan, MD warned that mask wearing has.. Get educated about what’s going on!
Get educated about what’s going on! What is The Great Reset?
What is The Great Reset?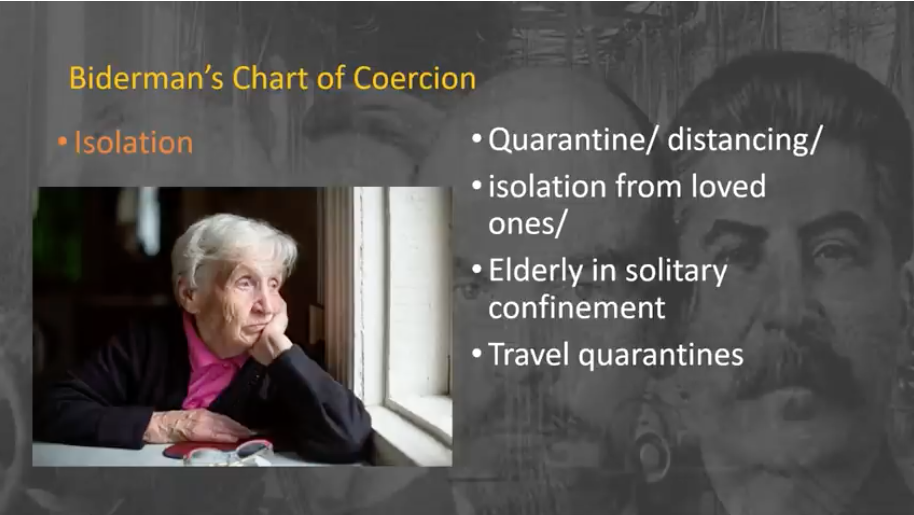 Isolation
Isolation
 Trusting the vaccine companies and their cohorts
Trusting the vaccine companies and their cohorts